Accuracy
Accuracy is the term used to describe the degree of conformity of a measured or calculated value to its actual or specified value.
Tolerance
This is the permissible range of variation of a characteristic from its nominal value.
Measurement uncertainty
Measurement uncertainty is the property of the result of a measurement which characterises the spread of the values that could reasonably be attributed to the result.
Where do errors and uncertainties come from?
As real measurements can never be made under perfect conditions, errors and uncertainties can come from:
| Source | Explanation |
| The measuring instrument | Instruments can suffer from errors including bias, changes due to ageing, wear, or other forms of drift, poor readability, and noise. |
| The item being measured | The item being measured may not be stable, for example measuring the size of an ice cube in a warm room. |
| The measurement process | The measurement itself may be difficult to make. For example, measuring the weight of a lively animal presents particular difficulties in getting the subject to cooperate. |
| 'Imported' uncertainties | Calibration of the instrument has an uncertainty which is then built into the uncertainty of the measurements made. |
| Operator skill | Some measurements depend upon the skill and judgment of the operator. One person may be better than another at the delicate work of setting up a measurement, or at reading fine detail by eye. The use of an instrument such as a stopwatch depends on the reaction time of the operator. (Gross mistakes are a different matter and are not to be accounted for as uncertainties). |
| Sampling issues | The measurements taken must be properly representative of the process being assessed. For example, do not place a thermometer on a wall near an air conditioning outlet to find the temperature at a workbench. |
| The environment | Temperature, air pressure, humidity and many other conditions can affect the measuring instrument or the item being measured. |
Accounting for errors
Where the size and effect of an error are known, for example from a calibration certificate, a correction can be applied to the measurement result. However, in general, uncertainties from each of the above sources (as well as from other sources) would be individual 'inputs' contributing to the overall uncertainty of the measurement.
The accuracy of the certificate on your gas mixture
When considering the 'accuracy' of a gas mixture we need to ask if we are looking at the accuracy of the filling or the accuracy of the certification.
Filling accuracy relates to how good the operator and equipment are at putting the components of the required mixture into the cylinder as close to the requested value as possible.
Filling
Gas manufacturers employ two main methods to cylinders in the right proportions for the gas mixture requested by the customer, these are:
- volumetrically, i.e. filling by putting a measured pressure of each component into the cylinder
- gravimetrically, i.e. weighing the gas, in proportion
Volumetric (to ISO 6144 standard)
Gas mixtures may be filled by putting a measured pressure of each component into the cylinder. This is known as volumetric filling.
For example, 10% nitrogen in oxygen, final pressure 200 bar
Partial pressure of nitrogen filled = 20 bar
Partial pressure of oxygen filled = 180 bar
| Component level | Filling tolerance (relative) |
| >=1% | +/-5% |
| >=10ppm <1% | +/-10% |
| >=1ppm <10ppm | +/-20% |
| <1ppm | +/-50% |
Note 1% = 10,000 ppm
Volumetrically prepared mixtures are certified by analysis.
Gravimetric (to ISO 6142 standard)
Gas mixtures may be filled by weighing the component gases. This is known as gravimetric filling.
For the highest filling-accuracies gas mixtures can be prepared on a Voland beam balance or on a set of mass comparators.
BOC will guarantee a filling tolerance of better than +/- 1% relative to the nominal concentration of each component or +/- 2 ppm, whichever is the greater.
As a general rule with percentage level mixtures, the relative accuracy of the fill is within +/- 0.25%.
Gravimetrically prepared mixtures are often certified without further work after filling, but may be analysed to generate the certificate.
Certification (see also Certification and Traceability)
The key difference between certifying by weight and certifying by analysis is that in the latter case there is validation by analysis of the stability and the homogeneity of the gas mixture.
The advantage of filling by weight is that for mixtures where the minor component is greater than around 0.5% of the mixture it is possible to offer a better filling tolerance, as the degree of control of the process is increased (see above). Below this level, volumetric filling is often a more effective methodology.
Tolerance is governed by what is practically achievable within the constraints imposed by man and machine. Uncertainty is the summation of all errors inherent within the process of making a mixture.
Both activities have a level of uncertainty and tolerance (i.e. a permissible range of variation) which can be calculated and therefore related to the level of accuracy of each process.
Relative or absolute accuracy?
Accuracy can be quoted in two ways:
- relative accuracy. This quotes the proportion of the error to the whole value (i.e. 14ppm +/- 10%)
- absolute accuracy. Absolute accuracy means that the quantity of the error is quoted (i.e. 14ppm +/- 1.4ppm)
BOC normally quotes the relative accuracy, but will clearly mark where it does not.
Confusion may occur when comparing certificates which do not state the method of expression used, for example 1ppm +/- 0.2ppm looks more impressive than 1ppm +/-20%
Example
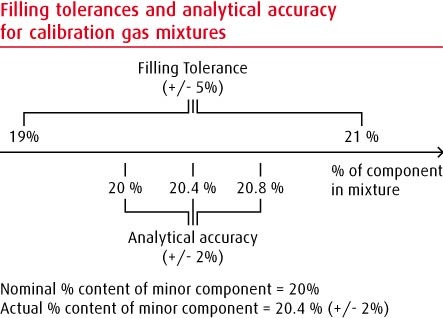
20% oxygen in nitrogen:
A filling accuracy of +/- 5% relative, means that the actual filled value of oxygen will be between 19% and 21% i.e. the mixture will be filled within +/- 5% of the required value.
If the requested level of certification is a Beta certificate then the result will have a certification accuracy of +/- 2% relative.
If, during analysis by BOC, the analytical instrument gives a result of 20.4%, then BOC will certify that the mixture will have a concentration between 20.0% and 20.8%.
Certification accuracy is often quoted by the manufacturers to be the same as filling accuracy. This is true where the mixture is gravimetrically filled, i.e. where the components are added by weight of addition, and no analysis of the resulting mixture is carried out.
BOC do not, as a rule, follow this methodology for calibration gases. What you put into a cylinder may not be what comes out.
To confirm that the contents have mixed completely and that the mixture is stable for most BOC mixtures analysis of the contents is carried out.